【輸入品・未使用】Medicines and Risk/Benefit Decisions (CMR Workshop Series)
(税込) 送料込み
商品の説明
【中古】【輸入品・未使用】
Medicines and Risk/Benefit Decisions (CMR Workshop Series)
【メーカー名】
Springer
【メーカー型番】
【ブランド名】
Springer
【商品説明】
Medicines and Risk/Benefit Decisions (CMR Workshop Series)
当店では初期不良に限り、商品到着から7日間は返品を 受付けております。
こちらは海外販売用に買取り致しました未使用品です。
買取り致しました為、中古扱いとしております。
他モールとの併売品の為、完売の際はご連絡致しますのでご了承下さい。
速やかにご返金させて頂きます。
ご注文からお届けまで
1、ご注文⇒ご注文は24時間受け付けております。
2、注文確認⇒ご注文後、当店から注文確認メールを送信します。
3、配送⇒当店海外倉庫から取り寄せの場合は10~30日程度でのお届けとなります。
国内到着後、発送の際に通知にてご連絡致します。
国内倉庫からの場合は3~7日でのお届けとなります。
※離島、北海道、九州、沖縄は遅れる場合がございます。予めご了承下さい。
お電話でのお問合せは少人数で運営の為受け付けておりませんので、メールにてお問合せお願い致します。
営業時間 月~金 10:00~17:00
お客様都合によるご注文後のキャンセル・返品は
お受けしておりませんのでご了承下さい。13272円【輸入品・未使用】Medicines and Risk/Benefit Decisions (CMR Workshop Series)本・雑誌・コミックその他Benefit-Risk Balance for Marketed Drugs: Evaluating Safety SignalsMedicines and Risk/Benefit Decisions (CMR Workshop Series): Centre
Medicines and Risk/Benefit Decisions (CMR Workshop Series)
Benefit-Risk Balance for Marketed Drugs: Evaluating Safety Signals
An integrated benefit-risk assessment of cobalt-containing alloys
粮食及农业状况2020
Clinical Risk Management: Enhancing Patient Safety, 2nd Edition
EMA and EATRIS Present: Scientific advice for advanced therapy
20180611202740_61580 | PDF
CMRT 6.31 版本可供下载- SiliconExpert
Additional Risk Minimisation Measures for Medicinal Products in
MDR / EUDAMED - Advanxa
CTD的问题-有问必答-品职教育专注CFA ESG FRM CPA 考研等财经培训课程
The Public Health Emergency Medical Countermeasures Enterprise: Innovative Strategies to Enhance Products from Discovery Through Approval: Workshop
Improved clinical investigation and evaluation of high-risk
Product Classification Determination Request - Product
Medical Devices - CIRS Group
Additional Risk Minimisation Measures for Medicinal Products in
Yanfeng Supplier Standard Manual
How to regulate Drug-Device Combination Products? (Article 117)
Medical device submissions: Placing a medical device on the market
MEDICAL DEVICES AND MDR: SUSTAINABLE SOLUTIONS FOR SURVEILLANCE
虛擬現實 Virtual Reality: 最新的百科全書、新聞、評論和研究
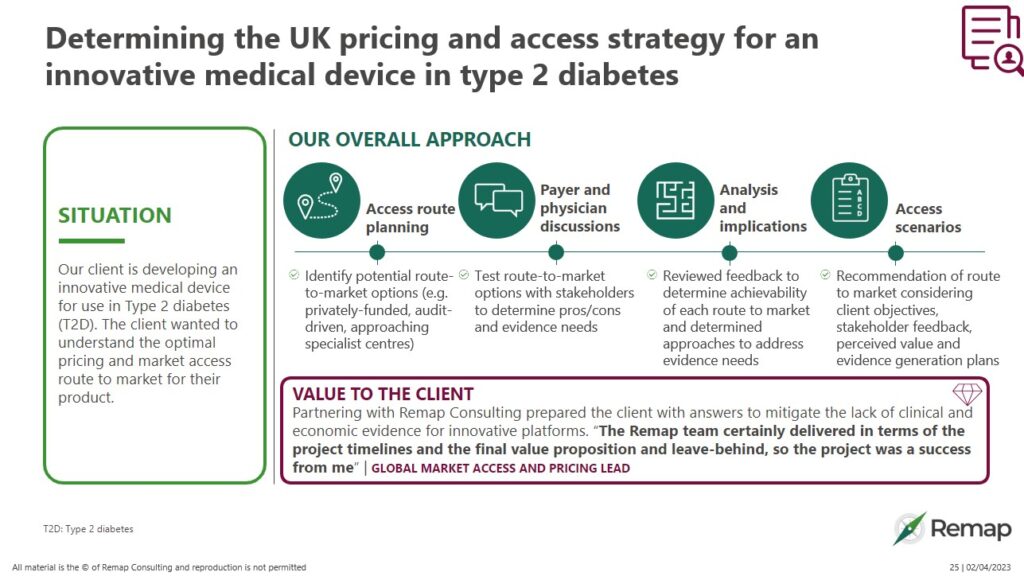
Determining the UK pricing and access strategy for an innovative
Procuring innovative ICT for patient empowerment and self
European Industrial Pharmacists Group (EIPG) guideline on
Medical device submissions: Placing a medical device on the market
Medication Errors: New EU Good Practice Guide on Risk Minimisation
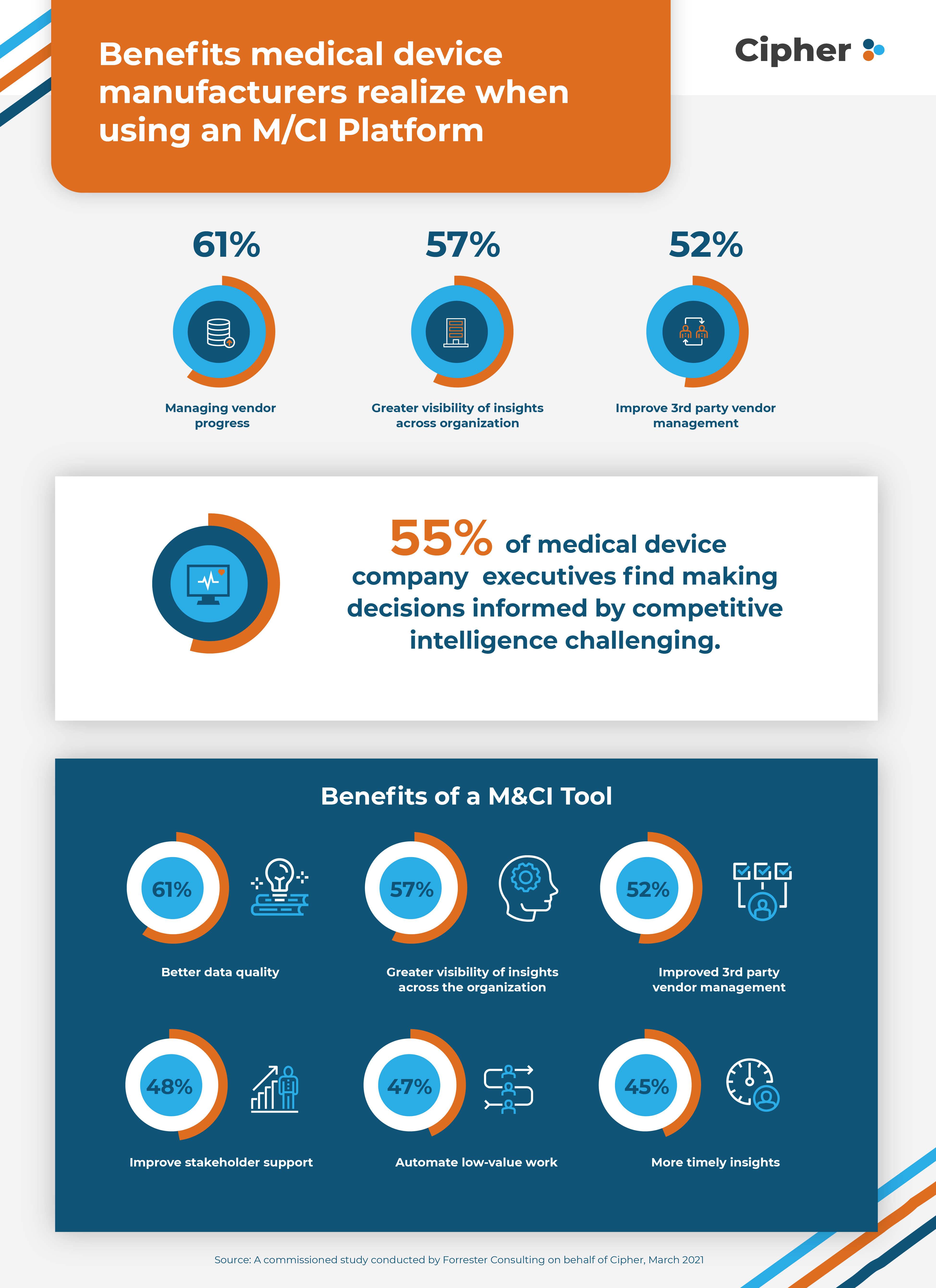
Benefits of an M/CI Platform for Medical Device Manufacturers
Medical device combination products: how to comply in the EU?
Medical Equipment | Precise Medical Comfort
How to design a regulatory strategy to optimize registration of
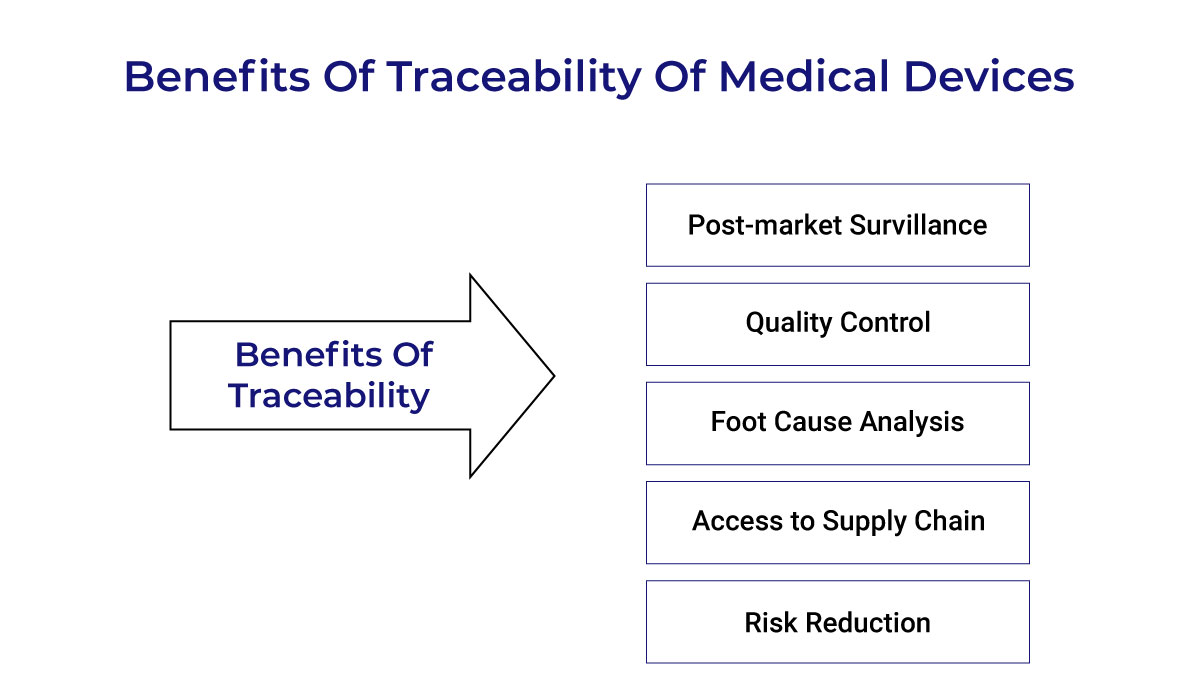
EU-MDR Traceability Requirements for Medical Devices (Ensuring
The combination of medical devices and medicinal products based on
The combination of medical devices and medicinal products
MDR Article 117: A New Implication for Drug-device Combination
Advanced Therapy Medicinal Products | Cevidra
Guidance on Use of Investigational Medical Devices in Human
Frontiers | The regulatory challenges of innovative customized
Medical device combination products: how to comply in the EU?
Implementing IDMP to improve patient safety and facilitate better
商品の情報
メルカリ安心への取り組み
お金は事務局に支払われ、評価後に振り込まれます
出品者
スピード発送
この出品者は平均24時間以内に発送しています